The Virtual Labs
The Virtual Physiology teaching tools come with detailed tutorials and protocol forms.
Preparation videos in high resolution are on this website and can also be linked to the labs.
Please check the system requirements.
SimHeart
SimHeart offers a virtual laboratory for recordings of heart contractions in the Langendorff set-up in response on the physiologically most relevant transmitters (Adrenaline, Acetylcholine) and clinically widely used drugs like the ß-blocker Propanolol, the a-blocker Phentolamine, the muscarinic receptor antagonist Atropine, the Ca2+-channel blocker Verapamil and theheart-glycoside g-Strophantine.
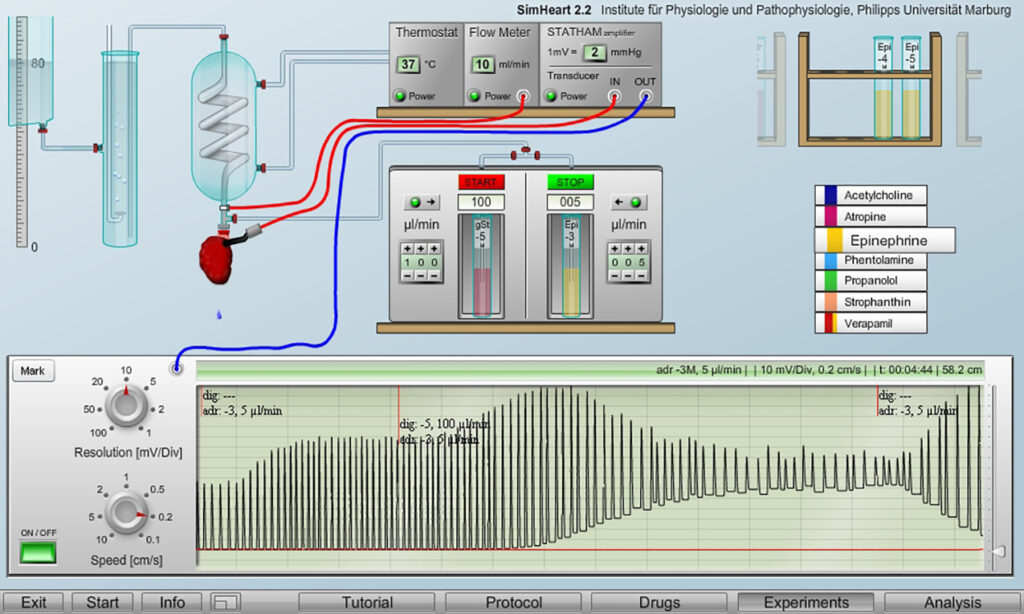
The example recording illustrate a situation that clinically should be avoided, namely the development of arrhythmias with a clear tendency towards a systolic heart block what can happen when a heart with already strong contractions, here induced by adrenalin, is additionally stimulated with heart glycosides.
The substances can be applied to the isolated heart in a broad range of freely selectable concentrations and in all combinations. Mathematical algorithms simulate the physiologically appropriate reactions – also under unusual combinations of drugs.
The force of the heart contractions is continuously recorded on a chart recorder. Parts of the recordings can be selected and stored for later data analysis.
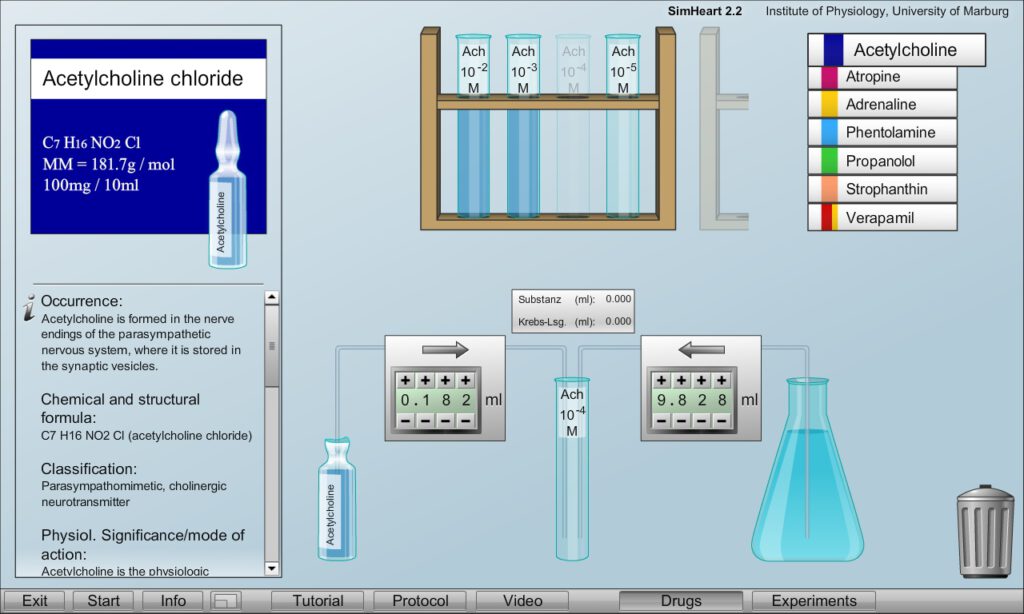
In a separate drug laboratory the correct adjustment of the desired drug solutions can be trained by diluting high concentrated drugs from commercially available vials in physiological solution (Krebs solution). The gravimetric concentration and molecular weight are indicated on the ampoule packages to calculate the correct dilution – which will be checked.
FEATURED EXPERIMENTS
- Effects of the physiological transmitters Adrenaline and Acetylcholine on frequency and amplitude of heart contractions (inotroph and chronotroph effects)
- Effects of competitive receptor blockers Propranolol, Phentolamine and Atropine
- Comparison with non-competitive inhibition by the Ca2+-channel blocker Verapamil
- Strengthening of the contractions by the heart-glycoside g-Strophantine
- Constructing dose-response curves and comparing their alterations of by competitive and non-competitive inhibitors
- Induction and treatment of arrhythmias and heart blocks in systole and diastole
FOR DETAILS SEE
TUTORIAL
PROTOCOL
VIDEO
SYSTEM REQUIREMENTS
DEMO VERSION
SimVessel
SimVessel offers a virtual laboratory for the examination of smooth muscle contractions from blood vessels and the intestine in response on physiologically relevant transmitters and clinically widely used drugs like receptor antagonists or the Ca channel blocker verapamil The here simulated preparations are from the antrum of the rat stomach and the aorta of the same specimen.
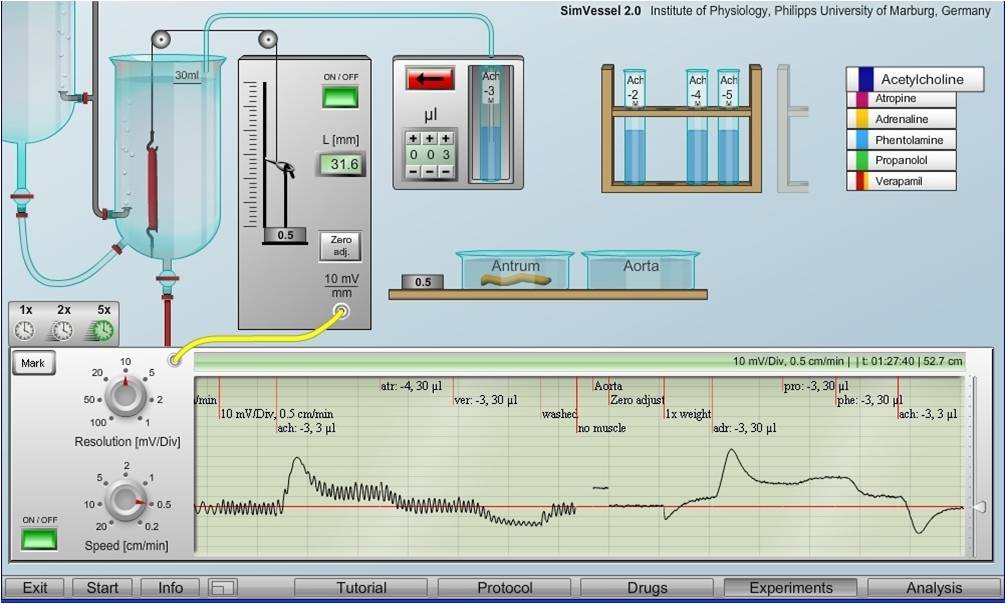
The example recordings compare different, partly opposite effects of diverse transmitters and drug on smooth muscles of the intestine (periodic contractions) and blood vessels (only tonic contractions), both showing phasic reactions on substance application with subsequent adaptation.
The experiments can be done with muscle stripes, placed in an organ bath to which diverse substances can be added. These include the physiological transmitters Adrenaline and Acetylcholine) as well as their competitive receptor antagonists phentolamine, propranolol and atropine. Additionally, the Ca-channel blocker verapamil can be applied and weights can be used to examine the effect of muscle stretching (Bayliss effcct).
The drugs can be added in freely selectable concentration in a broad range, also repeatedly, and in all combinations. Physiologically based mathematical algorithms calculate the thereby induced contractions which are documented on a chart recorder and can be stored for further date analysis.
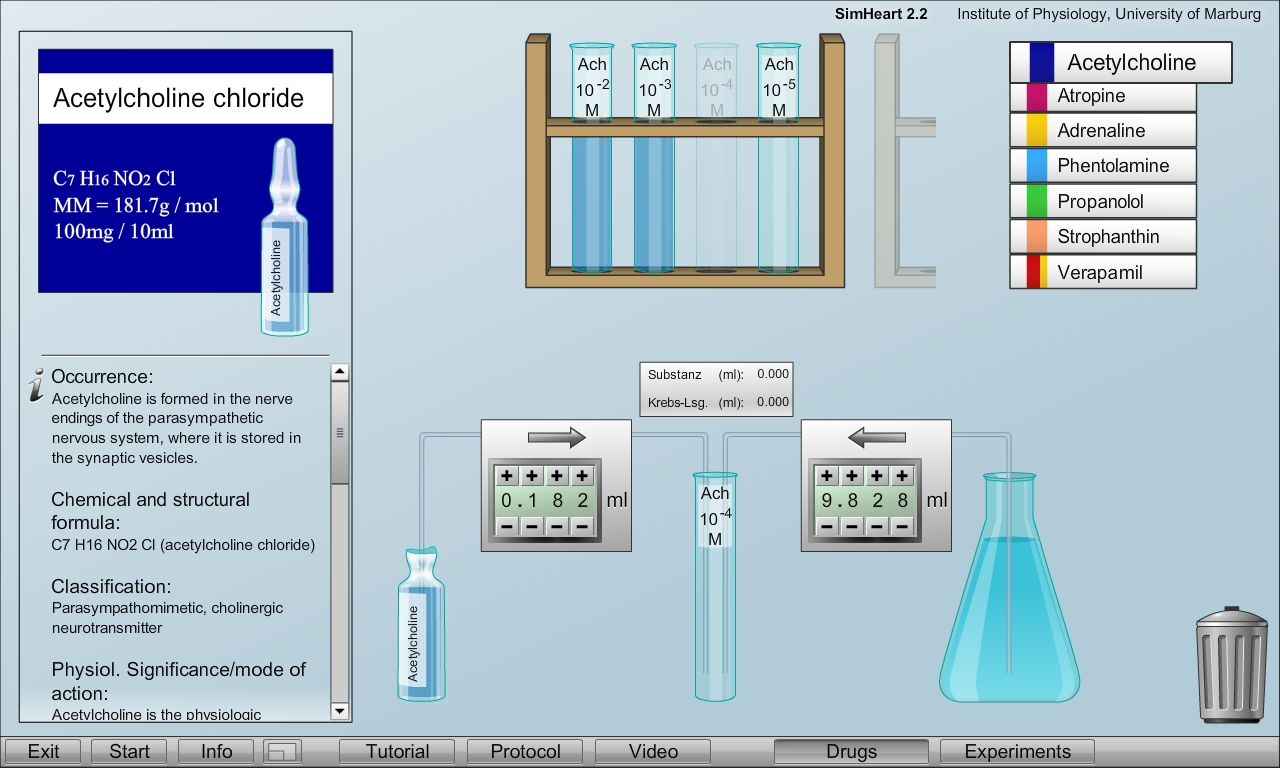
In addition to the experimental set-up a drug laboratory is offered to train how the correct the correct dilutions of the drugs for the experiments from commercially available ampoule with higher concentrations. Their concentration and molecular weights are indicated on the packages.
FEATURED EXPERIMENTS
- Comparison of phasic and tonic smooth muscle contractions
- Illustrating the effects of muscle stretching (Bayliss effect)
- Demonstrating opposite reactions of vessels and intestine on Adrenaline and Acetylcholine
- Examining the effects of the cholinergic receptor antagonist Atropine
- Comparing the effects of adrenergic α- and β-receptor antagonists (Phentolamine and Propranolol)
- Illustrating the effects of the Ca2+-channel blocker Verapamil
- Recording of dose-response curves of Adrenaline and Acetylcholine
- Demonstrating alterations of dose-response curves by competitive and non-competitive inhibitors
FOR DETAILS SEE
TUTORIALS
PROTOCOL
VIDEO
SYSTEM REQUIREMENTS
DOWNLOAD DEMO VERSION
SimMuscle
SimMuscle offers a fully equipped, realistically appearing laboratory on the computer screen to perform classical experiments with isolated nerve-muscle preparations of the frog.
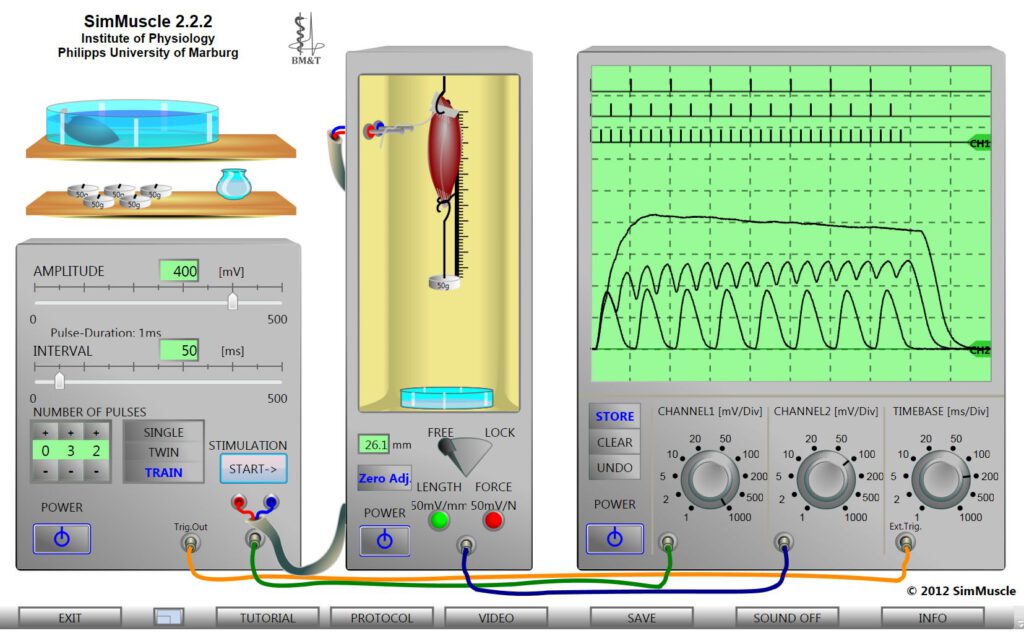
The oscilloscope screen shows example recording of muscle contractions, here changes of the muscle length, in response to different trains of voltage pulses inducing isolated twitches, incomplete and complete tetanic contractions depending on the intervals in which the pulses are applied.
All stimulation and recording parameters of the virtual devices are freely adjustable. Mathematical algorithms guarantee for the appropriated reactions of the virtual muscles, also considering the biological diversity of the preparations.
The experiments can be performed with two different already prepared nerve-muscle preparations placed in a Petri dish. One of the muscles can be hang it in the suspension apparatus. This includes a mechano-electrical convertertransforming changes of either the muscle force or muscle length (isometric or isotonic contractions), selectable by a toggle switch, into an electric potential. The muscle can be pre-stretched hanging one or more weights in the loop at which the muscle is fixed.
Muscle contractions are induced by current pulses delivered from a stimulation apparatus to the electrodes on which the nerve is placed. Single or double pulses as well as trains of stimuli of selectable amplitude and intervals can be applied. Muscle contractions are displayed together with the stimulus pulses on a dual beam storage oscilloscope with adjustablevoltage amplification and time base (via the rotary switches) and zero lines.
FEATURED EXPERIMENTS
- Inducing single twitches under isometric and isotonic conditions (locked and freely moveable muscles)
- Demonstrating recruitment of motor units with electric stimuli of increasing amplitude (single twitches)
- Demonstrating superposition of single twitches with double pulses of varying distance
- Applying trains of pulses of varying frequencies to compare isolated muscle twitches with incomplete and complete tetanic contractions
- Determining the resting tension curve (pre-stretching)
- Constructing the curves of isometric and isotonic maxima
- Demonstrating muscle fatigue
FOR DETAILS SEE
TUTORIALS
PROTOCOL
VIDEO
SYSTEM REQUIREMENTS
DOWNLOAD DEMO VERSION
SimNerv
SimNerv offers a fully equipped, realistically appearing laboratory on the computer screen for the induction and recording of compound action potentials. The experiments are performed with isolated preparations of the frog’s sciatic nerve which is particularly well suited illustrate the particular features and critical aspects of these clinically most relevant recordings of any electrical activity.
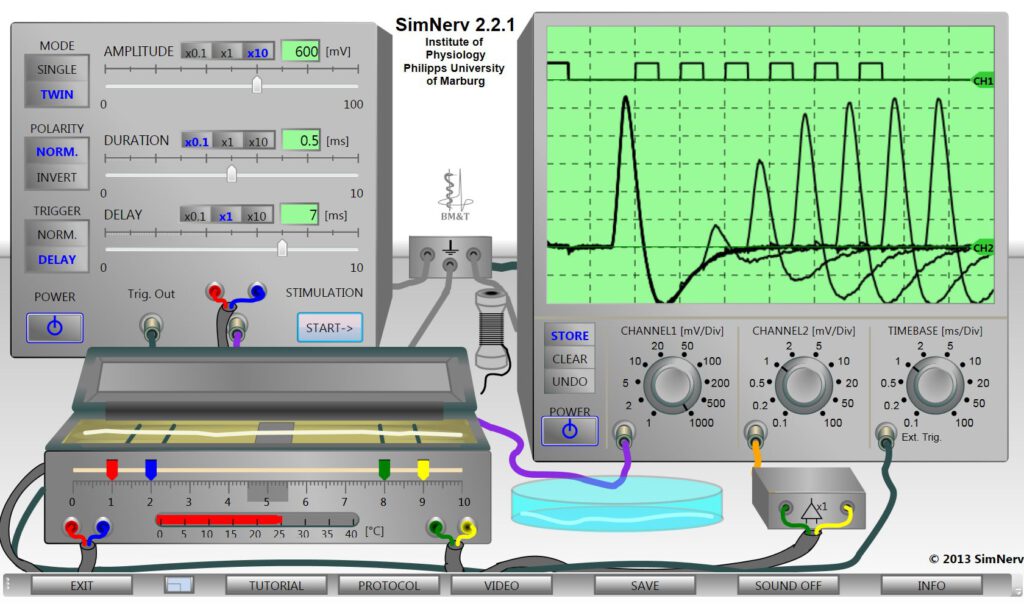
The example recordings on the oscilloscope screen illustrate the alteration of action potential amplitude in the refractory period in response on double pules of varying delay.
The “SimNerv” laboratory includes a stimulator, an oscilloscope, a recording chamber and a Petri dish with two already prepared nerves. All stimulation and recording parameters of the virtual devices are freely adjustable. Mathematical algorithms guarantee for the appropriated reactions of the virtual nerves under all stimulus conditions, also considering the biological diversity of the preparations.
For the experiments one of the nerves has to be taken out of the Petri-dish (by mouse click) and placed on the electrodes in the recording chamber (open and close with click on the lid) The position of the electrodes can be changed and the temperature of the recording chamber can be adjusted. A thread can be taken to set a nerve ligature (fully reversible).
The stimulator delivers voltage pulses of variable AMPLITUDE and DURATION. When TWIN are selected also the DELAY between the onsets of two successive stimuli can be adjusted. The POLARITY switch allows to invert the direction of the current flow corresponding to an exchange of the stimulus electrodes.
The simple double-beam storage oscilloscope displays the stimulus (channel 1), simultaneously with the compound action potential (channel 2) measured as the potential difference between the two recording electrodes by means of a differential amplifier. The sensitivity of both oscilloscope channels (mV/DIV) as well as their common time-base (ms/DIV) can be adjusted via the rotary switches.
FEATURED EXPERIMENTS
- Stimulus-response curve: recruitment of nerve fibres on increasing stimulus amplitudes
- Strength-duration curve, rheobase, chronaxy: the impact of stimulus duration
- Refractory period and anode break potentials: physiological and clinical relevance of Na+-channel inactivation:
- Conduction velocity and the effect of temperature changes
- Recordings of bi- and mono-phasic action potentials using reversible nerve ligatures
- Demonstrating the clinically most relevant impact of appropriate electrode positions
- Understanding the difference between intra- and extracellular recordings
FOR DETAILS SEE
TUTORIALS
PROTOCOL
VIDEO
SYSTEM REQUIREMENTS
DOWNLOAD DEMO VERSION
SimNeuron
SimNeuron offers virtual laboratories for voltage-clamp and current-clamp experiments in an easy to overlook lab design towards a better understanding of the relations between ion currents and membrane potentials including a neuron editor showing the mathematical equations also allowing modification and the neuron parameters.
The recordings show action potentials developing in response to current injection of increasing strength (left, current-clamp lab) and the time course of membrane currents (Na- and K currents) when the neuron is clamped to three different membrane potentials (right, voltage-clamp lab with RC compensation).
The labs come along with a so-called “Standard Neuron” and a “Pacemaker Neuron” both of pre-defined parameters. Additional neurons with random parameters values can be obtained. The neuron editor allows to generate and safe own neurons with self-defined membrane parameters.
The current-clamp lab is for recordings of membrane potential changes on external currents and the voltage-clamp lab is for recording of ion-currents when the neuron is clamped to defined membrane potentials.
Direct comparison of voltage-clamp and current clamp data from the same neuron can be of great help for a better understanding how neuronal sensitivity and activity is determined by ion channels’ activation and inactivation characteristics. Use of the neuron editor can become of particular value towards a deeper understanding of the consequences of ion channel modifications and alterations of passive membrane properties on the neuron’s dynamics.
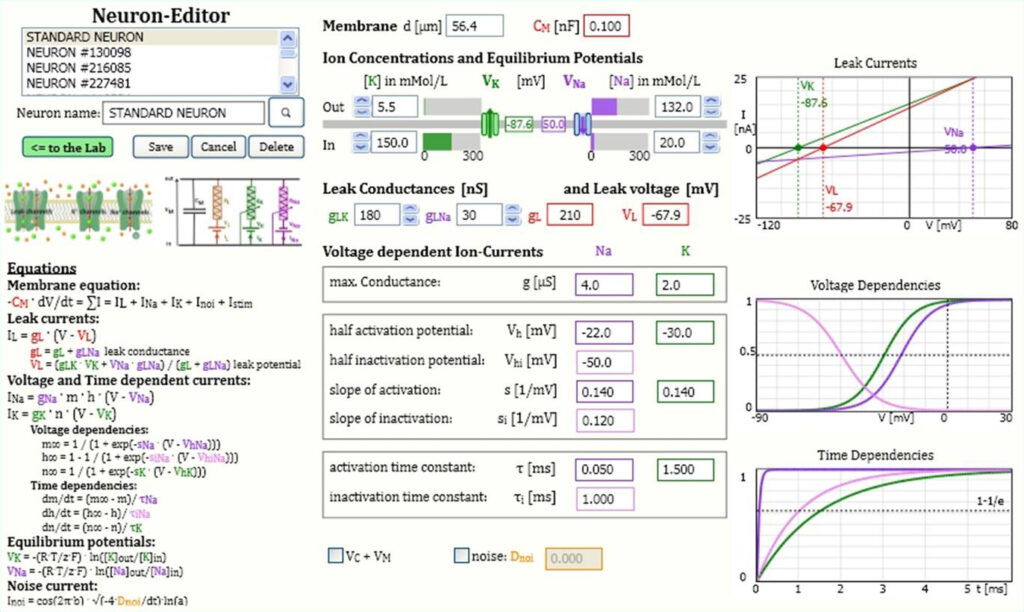
The figure shows the neuron editor with the full set of mathematical equations and numerical values of all neuron parameters. All parameter values can be changed, reflected in the corresponding diagrams of leak currents or voltage and time dependent activation and inactivation.
FEATURED EXPERIMENTS
– in the Current-Clamp Lab (action potential recordings)
- Determine the threshold of action potential generation
- Examine the effects of amplitude and duration of the current stimulus
- Induction of a series of action potentials of different frequencies
- Comparison of action potentials with local potentials and purely passive potential changes
- Examine the effects of the Na-channel blocker TTX and K+-channel blocker TEA
- Display and explain the alterations of ionic conductances and currents during an action potential (see also current-voltage curves)
– in the Voltage-Clamp Lab (ion current recordings)
- Determine the threshold of action potential generation
- Examine the effects of amplitude and duration of the current stimulus
- Induction of a series of action potentials of different frequencies
- Comparison of action potentials with local potentials and purely passive potential changes
- Examine the effects of the Na-channel blocker TTX and K+-channel blocker TEA
- Display and explain the alterations of ionic conductances and currents during an action potential (see also current-voltage curves)
– with use of the Neuron Editor
- Examine the effects of different membrane parameters on the neuron’s sensitivity, e.g. with alterations of voltage- and time-dependencies of ion current activation and inactivation or modifications of passive membrane properties like leak conductances, ion concentrations, etc.
- Take a neuron with originally constant resting potential, e.g. the Standard neuron, and try to induce pacemaker activity (repetitive firing) with appropriate membrane parameter alterations.
- Try to find out which parameter modifications are best suited to induce pacemaker activity and which other parameter changes might be the most effective ones to stabilize neurons’ membrane potentials.
- Design and safe your own neurons of your ptreferred characteristics
Pre-Settings:
In fully licensed versions there is the possibility to select to which specific features of the program the students shall have access. This can be done in so-called pre-settings window which you can open from the labs via the SETTINGS button in the switch bank. In the pre-settings of demo versions most functions are enabled.
FOR DETAILS SEE
TUTORIALS
PROTOCOL
VIDEO
SYSTEM REQUIREMENTS
DOWNLOAD DEMO VERSION
